With medical implant manufacturing poised for significant growth as techniques and materials continually improve, what are the major industry trends and developments that companies in the space need to know?
The healthcare industry is booming.
There are more seniors than ever before—according to the United Nations, the percentage of people 65 and older is growing faster than all other age groups—and as multinational medical product giant Johnson & Johnson points out, “People over the age of 65 years use approximately seven times morehealthcare-related products and services than younger people.”
But medical technology is improving as well, spurring further growth.
In 2017, SmarTech Publishing predicted close to 3.2 million 3D-printed implants would be in use by 2026, a nearly tenfold increase. Shortly afterward, Forbes labeled medical devices a disruptive market that is expected to reach $410 billion just a few years from now. And a recent blog post from the Duquesne University School of Nursing listed genomics sequencing toolkits and AI-infused (artificial intelligence) wearables as important industry trends.
What does all this mean to manufacturers? If your shop is riding the medical express train, plenty. And if it hasn’t, it might be time to look for a way to climb aboard.
Though the list is far from complete and the opportunities plenty, here are five medical manufacturing trends to keep an eye on, along with some recommendations on what’s needed to compete in this highly lucrative yet demanding industry:
No. 1: Remote Monitoring
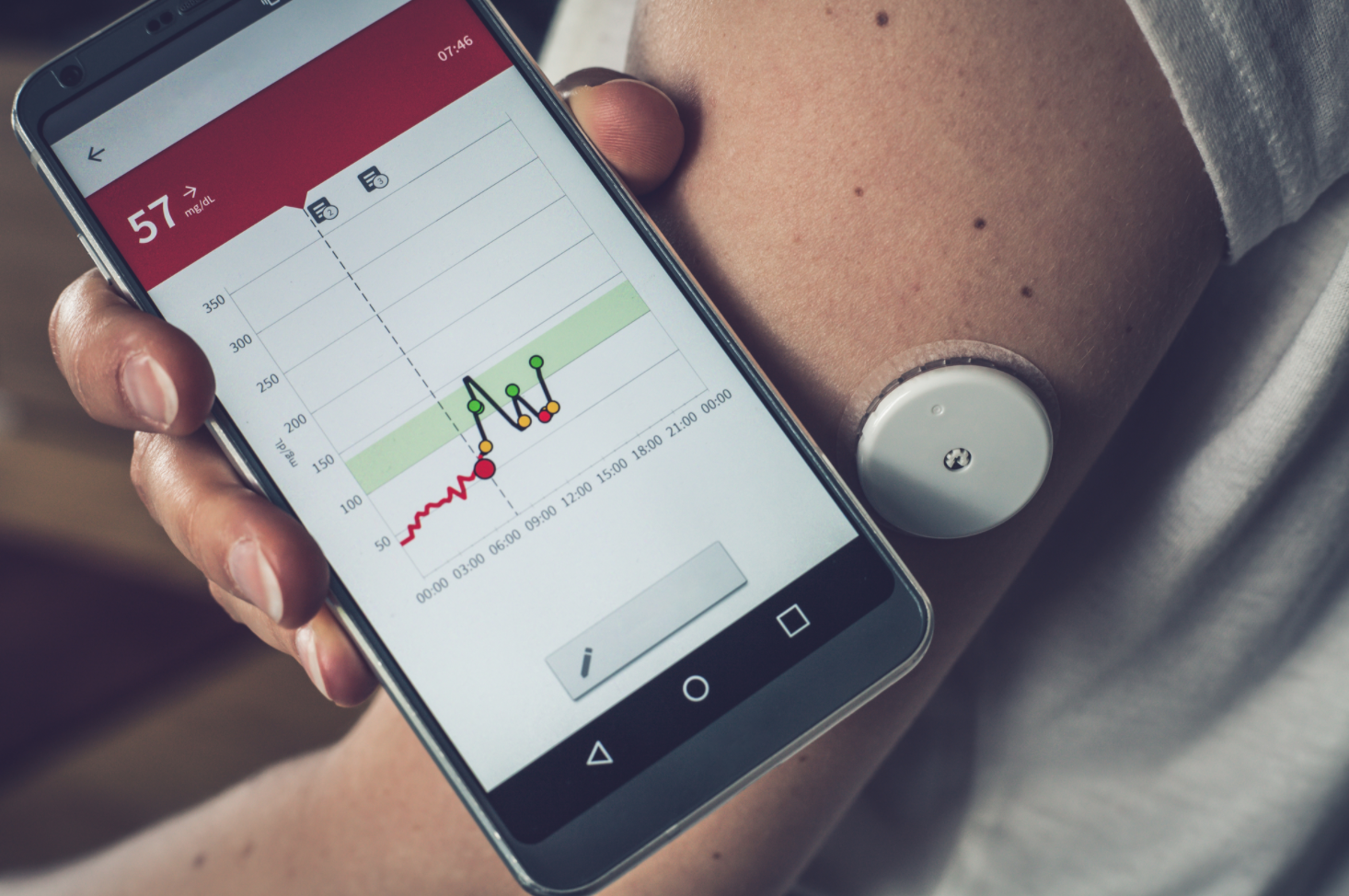
If you’ve ever had a knee or hip replacement, you know that the road to recovery can be many months long, the early stages of which (at least) require routine visits to a physical therapist. This is both costly and time-consuming, and since the start of the COVID-19 pandemic, has become risky as well. Wouldn’t it be cool if there were a sensor-based wearable device that could replicate what the physical therapist does, guiding the patient through a range-of-motion exercises and communicating progress back to the physician, all without having to visit a medical facility? Here’s an example of one such device, a cloud-based remote patient monitoring solution from Lewisville, Texas-based medical device manufacturer DJO LLC. Similar solutions exist for cardiac care, diabetic health, maternity monitoring and much more.
Read more: 5 Ways Manufacturers Can Use Data Analytics to Improve Efficiency
No. 2: Mechanobiologic Mechanisms

Speaking of knee and hip replacements, it seems that more is not always better. After studying decades of implant use and applying finite element analysis (FEA) to what some would call “overdesigned” orthopedic components, researchers have found that bones react better when subjected to appropriate amounts of stress and strain during the osseointegration stage, when the surrounding bone and tissues grow into the implant. One company leveraging this “mechanobiologic mechanism” concept is 4WEB Medical, which has developed truss implant technology that “may stimulate an osteogenic response to facilitate fusion.” Similar work is being done with dental implants, where surfaces of titanium screws are chemically modified to increase stability and improve patient outcomes. The takeaway? The software tools used to enhance performance in mechanical systems are now being applied to biological ones.
No. 3: Printing Human Parts
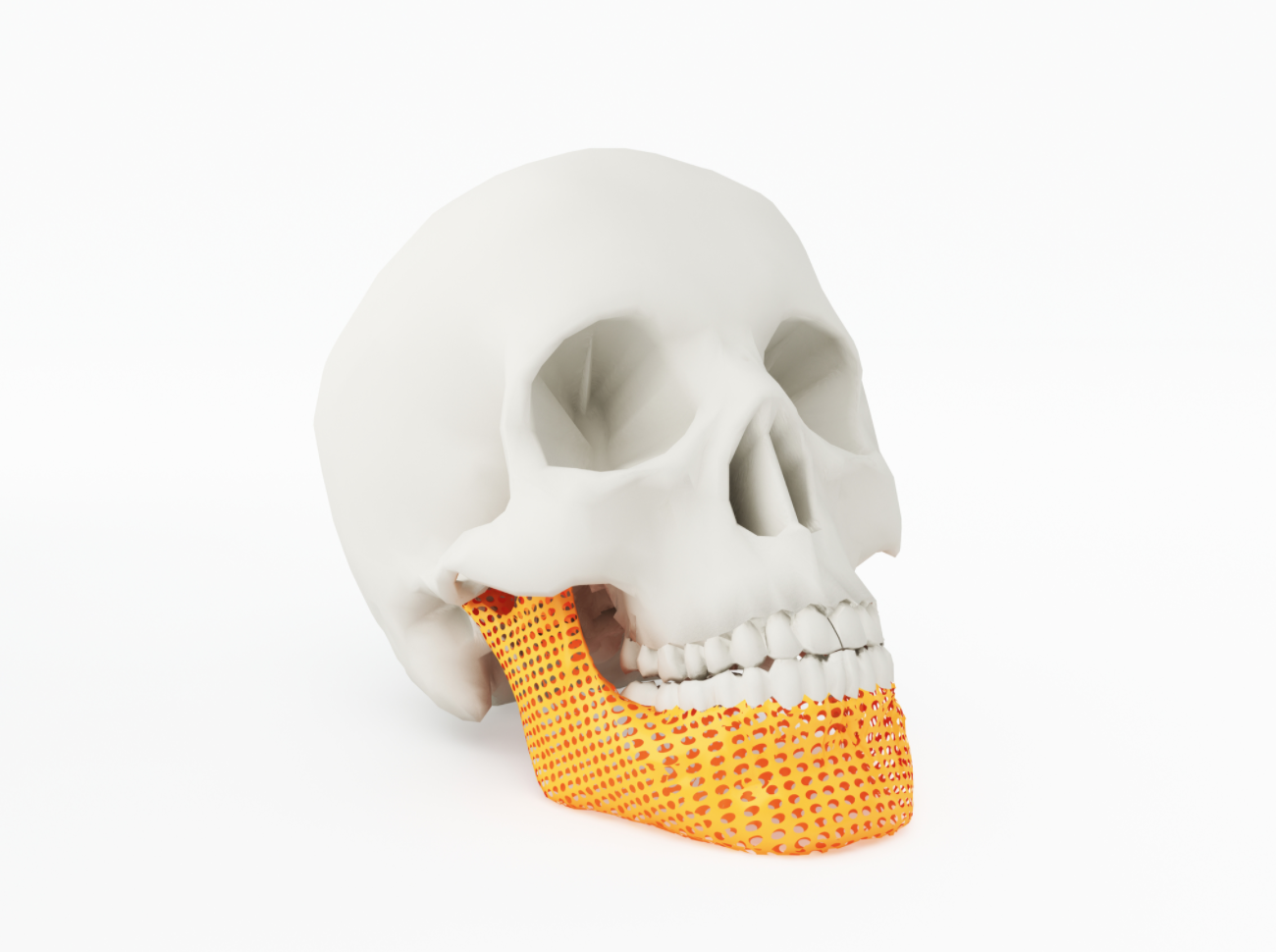
Of course, many of these new and improved replacement parts would not be possible without additive manufacturing, FEA software or not. Thanks to metal powder bed 3D-printing’s ability to create porous structures that closely mimic human bone, implants made with this technology are much more “osseo-friendly,” providing far greater staying power than their conventional counterparts. When coupled with CT scanning, physicians can image worn or broken bones and then manufacture patient-specific replacements. They can also scan the skull of an accident victim, for example, and 3D-print a titanium or PEKK (polyetherketoneketone) part that fits the damaged area perfectly. Taking that concept one step further, researchers have printed the first skin and cartilage replacements; many experts predict that organs and other complex body components are just a few years away.
Read more: Medical Machining: Cutting Tool Manufacturers Offer Insights, Advice for Success
No. 4: Robots in the Operating Room
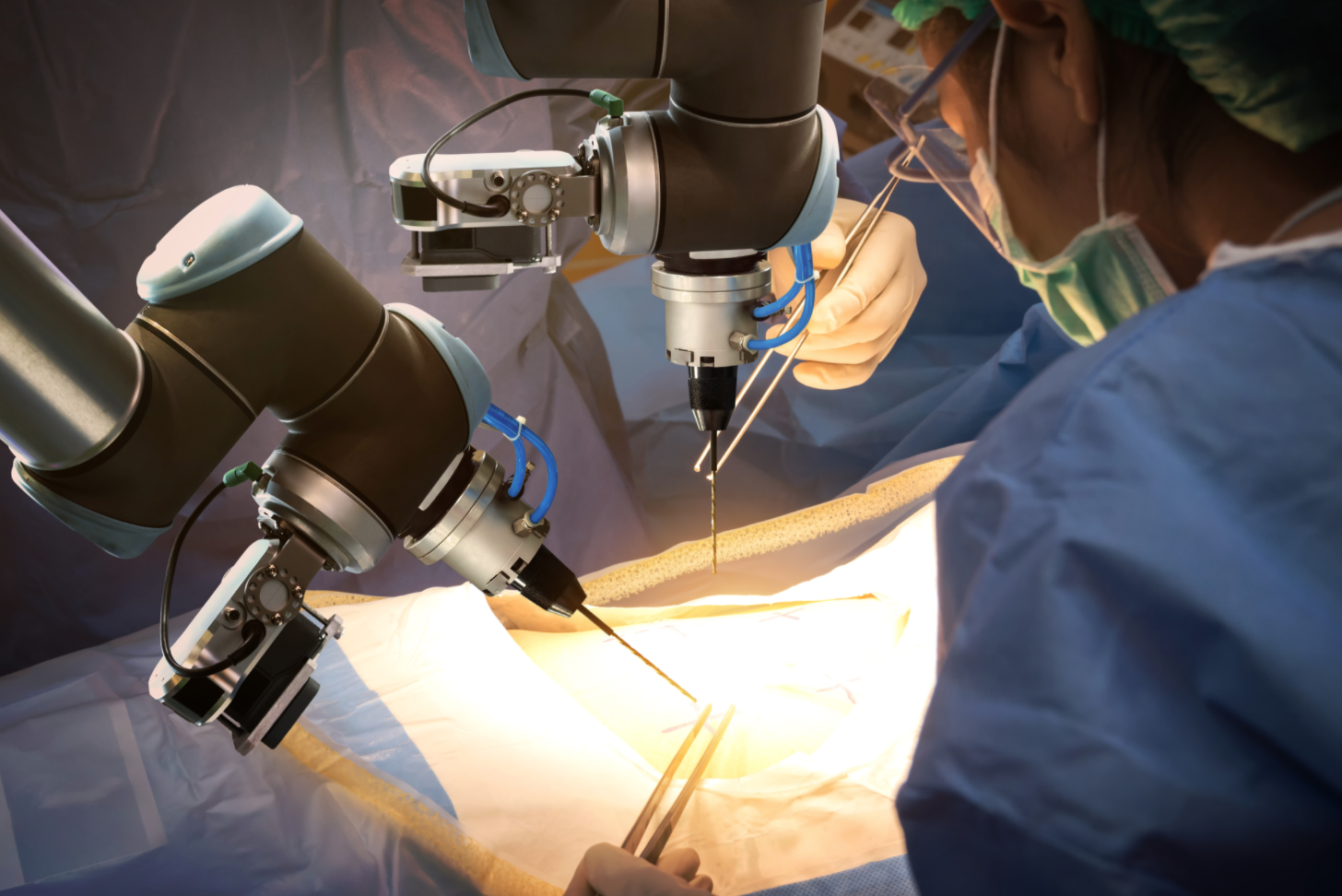
If you’ve had surgery over the past few years, it’s possible a robot held the instruments. If so, don’t be alarmed—there was also a skilled surgeon driving C3PO through its complex motions. Remote-controlled surgical robots are not only more precise than the human hand, they also have a greater range of motion and can fit into smaller areas. Because of this, robotic-assisted medical procedures are on the rise, and sales of these systems are expected to reach $20 billion by 2023. So is “extended reality,” which includes augmented, virtual and mixed reality systems. Here again, the use of this technology is climbing—Goldman Sachs predicts 3.4 million physicians and emergency medical technicians will be training on VR/AR systems or using them to treat patients by 2025.
Read more: How to Ring-Test, Mount, Balance and Store Your Grinding Wheels
No. 5: Synching Up

Fans of the 1970s television series The Six Million Dollar Man might recall that astronaut Steve Austin had superhuman strength, speed and vision thanks to his bionic limb and eye replacements. If Elon Musk has his way, that technology may soon become a reality. The founder of neural implant company Neuralink, Musk is working on ways to sync the human brain with prosthetic devices, computers and machines to provide thought-based control. He’s not alone. Members of the BrainGate consortium have been pursuing their own brain-computer interface (BCI) for nearly 20 years. Such “neuroprosthetics” could make it possible for those with spinal cord injuries, Lou Gehrig’s disease, and other disabilities leading to loss of speech or motion to live normal lives again.
Advancing Medical Technology
There are many more examples of advanced medical technology, available now and in the future. Digital twins of human patients will soon become commonplace, giving medical practitioners better data and decision-making capabilities. This is especially true given the increased use of wearables and remote monitoring systems, together with 5G networks that will expand medical reach into remote areas. As suggested earlier, genomics will play a leading role in disease detection and treatment, just as AI and machine learning will help physicians monitor large populations, identify health trends and potentially prevent epidemics.
Whether you’re a manufacturer looking to expand your customer base or a human looking for better, more affordable healthcare, the future looks very promising.
So You Want to Be a Medical Device Manufacturer?
There’s a lot to think about.
You’ll need to learn the differences between Class I, Class II and Class III devices. The first presents the lowest risk to patients and covers everything from bedpans to bandages. The most stringent—Class III—includes pacemakers, stents and orthopedic implants. Depending on the class, manufacturing any of these might require approval by the FDA and other regulatory agencies, as well as premarket approval. If not already in place, you should plan on implementing a robust quality management system, or QMS. This brief explanation is by no means complete. Start by looking at FDA 21 CFR Part 820 for manufacturing in the United States, and ISO 13485 for global markets. This article from the FDA provides some good background information.
You’ll also need the right equipment. Five-axis machining centers and Swiss-style CNC lathes are common in most medical shops, but it depends on the types of parts you plan to manufacture. As mentioned elsewhere, 3D printing has gained a significant foothold in this industry. The investment bar for additive manufacturing of polymer parts is much lower than that of the powder bed 3D printers used to produce metal implants.
In short, medical manufacturing is not for the faint of heart or shallow of pockets, but it’s certainly an important and growing market sector.
Thanks to Andy Christensen, business strategist for medical devices at Barnes Global Advisors, for his help with this article.

Related Articles

The Latest Tools and Techniques in Metal 3D Printing

HOW TO Put Innovation to Work for You

VIDEO: HOW TO Implement Grinding Solutions with Automation

Smart Restrooms: Companies See Value in IoT Connected Dispensers